|
|
|
| Lipogenic effects of androgen signaling in normal and malignant prostate |
Chui Yan Maha,b,Zeyad D. Nassara,b,Johannes V. Swinnenc,Lisa M. Butlera,b,*( ) )
|
a Adelaide Medical School and Freemasons Foundation Centre for Men's Health, University of Adelaide, Adelaide, Australia
b South Australian Health and Medical Research Institute, Adelaide, Australia
c KU Leuven- University of Leuven, LKI- Leuven Cancer Institute, Department of Oncology, Laboratory of Lipid Metabolism and Cancer, Leuven, Belgium |
|
|
|
|
Abstract Prostate cancer is an androgen-dependent cancer with unique metabolic features compared to many other solid tumors, and typically does not exhibit the “Warburg effect”. During malignant transformation, an early metabolic switch diverts the dependence of normal prostate cells on aerobic glycolysis for the synthesis of and secretion of citrate towards a more energetically favorable metabolic phenotype, whereby citrate is actively oxidised for energy and biosynthetic processes (i.e. de novo lipogenesis). It is now clear that lipid metabolism is one of the key androgen-regulated processes in prostate cells and alterations in lipid metabolism are a hallmark of prostate cancer, whereby increased de novo lipogenesis accompanied by overexpression of lipid metabolic genes are characteristic of primary and advanced disease. Despite recent advances in our understanding of altered lipid metabolism in prostate tumorigenesis and cancer progression, the intermediary metabolism of the normal prostate and its relationship to androgen signaling remains poorly understood. In this review, we discuss the fundamental metabolic relationships that are distinctive in normal versus malignant prostate tissues, and the role of androgens in the regulation of lipid metabolism at different stages of prostate tumorigenesis.
|
|
Received: 08 May 2019
Available online: 20 July 2020
|
|
Corresponding Authors:
Lisa M. Butler
E-mail: lisa.butler@adelaide.edu.au
|
|
|
|
|
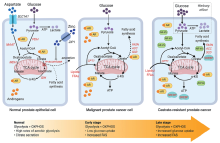
Metabolic landscape of prostate cancer progression (from normal prostate to malignant to metastatic/CRPC). Normal prostate epithelial cells exhibit high rates of aerobic glycolysis and low rates of oxidative phosphorylation. Glucose is used for citrate production and secretion, resulting in an impaired TCA cycle; this process is facilitated by zinc and aspartate through inhibition of m-aconitase and supply of metabolic precursors (i.e. oxaloacetate). Alternatively, citrate may be used for lipid biosynthesis through androgen-mediated activation of lipogenic enzymes. During malignant transformation, prostate cancer cells exhibit increased oxidative phosphorylation and hence, reactivate the TCA cycle to oxidize citrate for energy production. Instead of glucose, FFAs are the dominant bioenergetic substrates that feed into the TCA cycle for energy production. More importantly, de novo lipogenesis is enhanced at this stage of disease through the up-regulation of AR-regulated lipogenic enzymes. Despite initial positive responses to androgen-deprivation therapy, patients eventually progress to CRPC. AR signalling is maintained in CRPC; AR resistant mechanisms such as the AR-variants (AR-Vs) and/or indirect activation of alternative metabolic pathways (i.e. SREBP) play a role in driving the androgen-mediated lipogenic phenotype that may contribute to prostate cancer progression and treatment resistance. Notably, increased aerobic glycolysis or the Warburg effect is observed in advanced stages of the disease/CRPC. Words highlighted in red: AR-regulated genes. Thin/dotted lines: Baseline level; thick lines: Up-regulated. TCA, tricarboxylic acid cycle; PDH, pyruvate dehydrogenase; MAAT, aspartate aminotransferase; FASN, fatty acid biosynthesis; ACC, acetyl-CoA-carboxylase; SCD, stearoyl-CoA-desaturase; OXPHOS, oxidative phosphorylation; CRPC, castrate-resistant prostate cancer; AR, androgen receptor; FFAs, lipids or free fatty acids; MDH, malate dehydrogenase; α-KG, alpha ketoglutarate; AR-Vs, androgen receptor variants; CPT1, carnitine palmitoyltransferase 1; FAS, fatty acid synthesis. Created with BioRender.com.
|
|
|
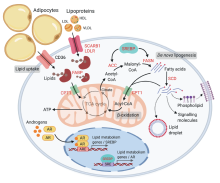
Overview of lipid metabolism in prostate cancer. Androgens regulate a number of lipid metabolic pathways in prostate cancer cells, including lipid uptake, biosynthesis and degradation. The AR directly (via binding of AR to the ARE) or indirectly (via activation of SREBP) regulates key lipid metabolic genes. Lipids can be derived from lipolysis of adipocytes or from circulating lipoproteins (LDL, HDL, VLDL). Alternatively, lipids can be synthesized endogenously by the cells via enzymes ACC and FASN. Androgens up-regulate lipid transporters that transport lipids or free fatty acids to the mitochondria for fatty acid oxidation (β-oxidation) or de novo lipogenesis. Free fatty acids are shuttled into the mitochondria as Acyl-CoAs by coupling with CPT1 (a mitochondrial transporter); a cyclical series of reactions result in the shortening of Acyl-CoA and the production of energy in the form of ATP via the TCA cycle. Citrate produced from the TCA cycle may also be cleaved to produce Acetyl-CoA, which is a precursor for de novo lipogenesis in the cytosol. Synthesized fatty acids may be further modified (i.e. degree of saturation via SCD) to generate a variety of lipids such as phospholipids that compose the plasma membranes, to promote lipid droplet production or to act as oncogenic signalling molecules to drive disease progression. Words in red = androgen-regulated genes. ARE, androgen response element; SRE, SREBP response element; AR, androgen receptor; LDL, low density lipoprotein; HDL, high density lipoprotein; VLDL, very low density lipoprotein; FASN, fatty acid biosynthesis; TCA, tricarboxylic acid cycle; SCD, stearoyl-CoA-desaturase; SREBP, sterol response element binding protein. Created with BioRender.com.
|
| [1] |
Gonthier K, Poluri RTK, Audet-Walsh é. Functional genomic studies reveal the androgen receptor as a master regulator of cellular energy metabolism in prostate cancer. J Steroid Biochem Mol Biol 2019. 191. 105367. https://doi.org/10.1016/j.jsbmb.2019.04.016.
doi: 10.1016/j.jsbmb.2019.04.016
pmid: 31051242
|
| [2] |
Barfeld SJ, Itkonen HM, Urbanucci A, Mills IG. Androgenregulated metabolism and biosynthesis in prostate cancer. Endocr Relat Cancer 2014; 21:T57-66. https://doi.org/10.1530/ERC-13-0515.
|
| [3] |
Butler LM, Centenera MM, Swinnen JV. Androgen control of lipid metabolism in prostate cancer: novel insights and future applications. Endocr Relat Cancer 2016; 23:R219-27. https://doi.org/10.1530/ERC-15-0556.
|
| [4] |
Itkonen HM, Brown M, Urbanucci A, Tredwell G, Ho Lau C, Barfeld S, et al. Lipid degradation promotes prostate cancer cell survival. Oncotarget 2017; 8:38264-75.
|
| [5] |
Tamura K, Makino A, Hullin-Matsuda F, Kobayashi T, Furihata M, Chung S, et al. Novel lipogenic enzyme ELOVL7 is involved in prostate cancer growth through saturated longchain fatty acid metabolism. Cancer Res 2009; 69:8133-40.
|
| [6] |
Lamont KR, Tindall DJ. Androgen regulation of gene expression. Adv Cancer Res 2010; 107:137-62.
|
| [7] |
Swinnen JV, Heemers H, van de Sande T, de Schrijver E, Brusselmans K, Heyns W, et al. Androgens, lipogenesis and prostate cancer. J Steroid Biochem Mol Biol 2004; 92:273-9.
|
| [8] |
Swinnen JV, Verhoeven G. Androgens and the control of lipid metabolism in human prostate cancer cells. J Steroid Biochem Mol Biol 1998; 65:191-8.
|
| [9] |
Costello LC, Franklin RB. Concepts of citrate production and secretion by prostate. 1. Metabolic relationships. Prostate 1991; 18:25-46.
|
| [10] |
Kavanagh JP. Sodium, potassium, calcium, magnesium, zinc, citrate and chloride content of human prostatic and seminal fluid. J Reprod Fertil 1985; 75:35-41.
|
| [11] |
Costello LC, Franklin RB. The intermediary metabolism of the prostate: a key to understanding the pathogenesis and progression of prostate malignancy. Oncology 2000; 59:269-82.
|
| [12] |
Hicks JJ, Martínez-Manautou J, Pedron N, Rosado A. Metabolic changes in human spermatozoa related to capacitation. Fertil Steril 1972; 23:172-9.
|
| [13] |
Arver S. Zinc and zinc ligands in human seminal plasma. III. The principal low molecular weight zinc ligand in prostatic secretion and seminal plasma. Acta Physiol Scand 1982; 116:67-73.
|
| [14] |
Tomlins AM, Foxall PJ, Lynch MJ, Parkinson J, Everett JR, Nicholson JK. High resolution 1H NMR spectroscopic studies on dynamic biochemical processes in incubated human seminal fluid samples. Biochim Biophys Acta 1998; 1379:367-80.
|
| [15] |
Searcy RL, Simms NM. A practical approach for acid-base characterization of human semen. Int J Fertil 1967; 12:329-34.
|
| [16] |
Ford WC, Harrison A. The role of citrate in determining the activity of calcium ions in human semen. Int J Androl 1984; 7:198-202.
|
| [17] |
Hori T, Masuda T, Kobayashi M, Kawakami E. Role of prostatic fluid in cooled canine epididymal sperm. Reprod Domest Anim 2017; 52:655-60.
|
| [18] |
Medrano A, Fernández-Novell JM, Ramió L, Alvarez J, Goldberg E, Montserrat Rivera M, et al. Utilization of citrate and lactate through a lactate dehydrogenase and ATPregulated pathway in boar spermatozoa. Mol Reprod Dev 2006; 73:369-78.
|
| [19] |
H?rk?nen P. Androgenic control of glycolysis, the pentose cycle and pyruvate dehydrogenase in the rat ventral prostate. J Steroid Biochem 1981; 14:1075-84.
|
| [20] |
Müntzing J, Varkarakis MJ, Saroff J, Murphy GP. Comparison and significance of respiration and glycolysis of prostatic tissue from various species. J Med Primatol 1975; 4:245-51.
|
| [21] |
Farnsworth WE. Testosterone stimulation of citric acid synthesis in the rat prostate. Biochim Biophys Acta 1966; 117:247-54.
|
| [22] |
H?rk?nen P, Isotalo A, Santti R. Studies on the mechanism of testosterone action on glucose metabolism in the rat ventral prostate. J Steroid Biochem 1975; 6:1405-13.
|
| [23] |
Santti RS, Villee CA. Hormonal control of hexokinase in male sex accessory glands. Endocrinology 1971; 89:1162-70.
|
| [24] |
Costello LC, Akuffo V, Franklin RB. Net citrate production by isolated prostate epithelial cells. Enzyme 1988; 39:125-33.
|
| [25] |
Banerjee S, Zare RN, Tibshirani RJ, Kunder CA, Nolley R, Fan R, et al. Diagnosis of prostate cancer by desorption electrospray ionization mass spectrometric imaging of small metabolites and lipids. Proc Natl Acad Sci USA 2017; 114:3334-9.
|
| [26] |
Costello LC, Franklin R, Stacey R. Mitochondrial isocitrate dehydrogenase and isocitrate oxidation of rat ventral prostate. Enzyme 1976; 21:495-506.
|
| [27] |
Costello LC, Franklin RB, Narayan P. Citrate in the diagnosis of prostate cancer. Prostate 1999; 38:237-45.
|
| [28] |
Costello LC, Franklin RB. Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate 1998; 35:285-96.
|
| [29] |
Costello LC, Feng P, Milon B, Tan M, Franklin RB. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis 2004; 7:111-7.
|
| [30] |
Franklin RB, Milon B, Feng P, Costello LC. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front Biosci 2005; 10:2230-9.
|
| [31] |
Costello LC, Franklin RB, Liu Y, Kennedy MC. Zinc causes a shift toward citrate at equilibrium of the m-aconitase reaction of prostate mitochondria. J Inorg Biochem 2000; 78:161-5.
|
| [32] |
Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J Biol Chem 1997; 272:28875-81.
|
| [33] |
Eliasson R. Cholesterol in human semen. Biochem J 1966; 98:242-3.
|
| [34] |
Costello LC, Liu Y, Franklin RB. Testosterone stimulates the biosynthesis of m-aconitase and citrate oxidation in prostate epithelial cells. Mol Cell Endocrinol 1995; 112:45-51.
|
| [35] |
Costello LC, Liu Y, Franklin RB. Testosterone and prolactin stimulation of mitochondrial aconitase in pig prostate epithelial cells. Urology 1996; 48:654-9.
|
| [36] |
Costello LC, Liu Y, Zou J, Franklin RB. Mitochondrial aconitase gene expression is regulated by testosterone and prolactin in prostate epithelial cells. Prostate 2000; 42:196-202.
|
| [37] |
Costello LC, Liu Y, Zou J, Franklin RB. The pyruvate dehydrogenase E1 alpha gene is testosterone and prolactin regulated in prostate epithelial cells. Endocr Res 2000; 26:23-39.
|
| [38] |
Franklin RB, Brandly RL, Costello LC. Mitochondrial aspartate aminotransferase and the effect of testosterone on citrate production in rat ventral prostate. J Urol 1982; 127:798-802.
|
| [39] |
Franklin RB, Brandly RL, Costello LC. Effect of inhibitors of RNA and protein synthesis on mitochondrial aspartate aminotransferase response to testosterone in rat ventral prostate. Prostate 1982; 3:637-42.
|
| [40] |
Franklin RB, Kahng MW, Akuffo V, Costello LC. The effect of testosterone on citrate synthesis and citrate oxidation and a proposed mechanism for regulation of net citrate production in prostate. Horm Metab Res 1986; 18:177-81.
|
| [41] |
Costello LC, Akuffo V, Franklin RB. Testosterone stimulates net citrate production from aspartate by prostate epithelial cells. Horm Metab Res 1988; 20:252-3.
|
| [42] |
Costello LC, Liu Y, Zou J, Franklin RB. Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J Biol Chem 1999; 274:17499-504.
|
| [43] |
Liu Y, Franklin RB, Costello LC. Prolactin and testosterone regulation of mitochondrial zinc in prostate epithelial cells. Prostate 1997; 30:26-32.
|
| [44] |
Juang HH, Costello LC, Franklin RB. Androgen modulation of multiple transcription start sites of the mitochondrial aspartate aminotransferase gene in rat prostate. J Biol Chem 1995; 270:12629-34.
|
| [45] |
Qian K, Franklin RB, Costello LC. Testosterone regulates mitochondrial aspartate aminotransferase gene expression and mRNA stability in prostate. J Steroid Biochem Mol Biol 1993; 44:13-9.
|
| [46] |
Franklin RB, Zou J, Yu Z, Costello LC. EAAC1 is expressed in rat and human prostate epithelial cells; functions as a highaffinity L-aspartate transporter; and is regulated by prolactin and testosterone. BMC Biochem 2006; 7:10. https://doi.org/10.1186/1471-2091-7-10.
doi: 10.1186/1471-2091-7-10
pmid: 16566829
|
| [47] |
Costello LC, Liu Y, Franklin RB. Prolactin specifically increases pyruvate dehydrogenase E1 alpha in rat lateral prostate epithelial cells. Prostate 1995; 26:189-93.
|
| [48] |
Franklin RB, Juang HH, Zou J, Costello LC. Regulation of citrate metabolism by androgen in the LNCaP human prostate carcinoma cell line. Endocrine 1995; 3:603-7.
|
| [49] |
Arunakaran J, Balasubramanian K, Srinivasan N, Aruldhas MM, Govindarajulu P. Interaction of androgens and prolactin on prostatic enzymes of the pyruvate-malate cycle involved in lipogenesis in castrated mature monkey, Macaca radiata. Cytobios 1992; 70:33-40.
|
| [50] |
Chen M, Zhang J, Sampieri K, Clohessy JG, Mendez L, Gonzalez- Billalabeitia E, et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat Genet 2018; 50:206-18.
|
| [51] |
Choi SM, Kam SC. Metabolic effects of androgen deprivation therapy. Korean J Urol 2015; 56:12-8.
|
| [52] |
Heemers H, Vanderhoydonc F, Roskams T, Shechter I, Heyns W, Verhoeven G, et al. Androgens stimulate coordinated lipogenic gene expression in normal target tissues in vivo. Mol Cell Endocrinol 2003; 205:21-31.
|
| [53] |
Arunakaran J, Aruldhas MM, Govindarajulu P. Effects of prolactin and androgens on the prostatic lipids of castrated mature bonnet monkeys. Prostate 1990; 17:247-60.
|
| [54] |
Arunakaran J, Aruldhas MM, Govindarajulu P. Influence of castration and testosterone propionate on prostatic and seminal vesicular lipids in mature monkeys. Indian J Physiol Pharmacol 1987; 31:184-9.
|
| [55] |
Nyden SJ, Williams-Ashman HG. Influence of androgens on synthetic reactions in ventral prostate tissue. Am J Physiol 1953; 172:588-600.
|
| [56] |
Warburg O. On the origin of cancer cells. Science 1956; 123:309-14.
|
| [57] |
Warburg O. über den stoffwechsel der carcinomzelle. Naturwissenschaften 1924; 12:1131-7.
|
| [58] |
DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv 2016; 2:e1600200. https://doi.org/10.1126/sciadv.1600200.
pmid: 27386546
|
| [59] |
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metabol 2008; 7:11-20.
|
| [60] |
Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11Cacetate, and 18F- or 11C-choline. J Nucl Med 2011; 52:81-9.
|
| [61] |
Li W, Cohen A, Sun Y, Squires J, Braas D, Graeber TG, et al. The role of CD44 in glucose metabolism in prostatic small cell neuroendocrine carcinoma. Mol Cancer Res 2016; 14:344-53.
|
| [62] |
Vaz CV, Alves MG, Marques R, Moreira PI, Oliveira PF, Maia CJ, et al. Androgen-responsive and nonresponsive prostate cancer cells present a distinct glycolytic metabolism profile. Int J Biochem Cell Biol 2012; 44:2077-84.
|
| [63] |
Lavallée E, Bergeron M, Buteau FA, Blouin AC, Duchesnay N, Dujardin T, et al. Increased prostate cancer glucose metabolism detected by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in localised gleason 8-10 prostate cancers identifies very high-risk patients for early recurrence and resistance to castration. Eur Urol Focus 2019; 5:998-1006.
|
| [64] |
Zacharias N, Lee J, Ramachandran S, Shanmugavelandy S, McHenry J, Dutta P, et al. Androgen receptor signaling in castration-resistant prostate cancer alters hyperpolarized pyruvate to lactate conversion and lactate levels in vivo. Mol Imaging Biol 2019; 21:86-94.
|
| [65] |
Bok R, Lee J, Sriram R, Keshari K, Sukumar S, Daneshmandi S, et al. The role of lactate metabolism in prostate cancer progression and metastases revealed by dual-agent hyperpolarized 13C MRSI. Cancers (Basel) 2019; 11:E257. https://doi.org/10.3390/cancers11020257.
|
| [66] |
Massie CE, Lynch A, Ramos-Montoya A, Boren J, Stark R, Fazli L, et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J 2011; 30:2719-33.
|
| [67] |
Swinnen JV, Van Veldhoven PP, Esquenet M, Heyns W, Verhoeven G. Androgens markedly stimulate the accumulation of neutral lipids in the human prostatic adenocarcinoma cell line LNCaP. Endocrinology 1996; 137:4468-74.
|
| [68] |
Nelson PS, Clegg N, Arnold H, Ferguson C, Bonham M, White J, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci USA 2002; 99:11890-5.
|
| [69] |
Ngan S, Stronach EA, Photiou A, Waxman J, Ali S, Buluwela L. Microarray coupled to quantitative RT-PCR analysis of androgen-regulated genes in human LNCaP prostate cancer cells. Oncogene 2009; 28:2051-63.
|
| [70] |
Rossi S, Graner E, Febbo P, Weinstein L, Bhattacharya N, Onody T, et al. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res 2003; 1:707-15.
|
| [71] |
Swinnen JV, Roskams T, Joniau S, Van Poppel H, Oyen R, Baert L, et al. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer 2002; 98:19-22.
|
| [72] |
Swinnen JV, Vanderhoydonc F, Elgamal AA, Eelen M, Vercaeren I, Joniau S, et al. Selective activation of the fatty acid synthesis pathway in human prostate cancer. Int J Cancer 2000; 88:176-9.
|
| [73] |
Kuhajda FP, Jenner K, Wood FD, Hennigar RA, Jacobs LB, Dick JD, et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci USA 1994; 91:6379-83.
|
| [74] |
Medes G, Thomas A, Weinhouse S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res 1953; 13:27-9.
|
| [75] |
Zadra G, Photopoulos C, Loda M. The fat side of prostate cancer. Biochim Biophys Acta 2013; 1831:1518-32.
|
| [76] |
Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J 2012; 279:2610-23.
|
| [77] |
Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care 2006; 9:358-65.
|
| [78] |
Zadra G, Loda M. Metabolic vulnerabilities of prostate cancer: diagnostic and therapeutic opportunities. Cold Spring Harb Perspect Med 2018; 8:a030569. https://doi.org/10.1101/cshperspect.a030569.
pmid: 29229664
|
| [79] |
Shurbaji MS, Kalbfleisch JH, Thurmond TS. Immunohistochemical detection of a fatty acid synthase (OA-519) as a predictor of progression of prostate cancer. Hum Pathol 1996; 27:917-21.
|
| [80] |
Luo X, Cheng C, Tan Z, Li N, Tang M, Yang L, et al. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer 2017; 16:76. https://doi.org/10.1186/s12943-017-0646-3.
doi: 10.1186/s12943-017-0646-3
pmid: 28399876
|
| [81] |
Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the acetyl-CoAcarboxylase- alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res 2005; 65:6719-25.
|
| [82] |
Beckers A, Organe S, Timmermans L, Scheys K, Peeters A, Brusselmans K, et al. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res 2007; 67:8180-7.
|
| [83] |
Staubach S, Hanisch FG. Lipid rafts: signaling and sorting platforms of cells and their roles in cancer. Expert Rev Proteomics 2011; 8:263-77.
|
| [84] |
Swinnen JV, Van Veldhoven PP, Timmermans L, De Schrijver E, Brusselmans K, Vanderhoydonc F, et al. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem Biophys Res Commun 2003; 302:898-903.
|
| [85] |
Deep G Schlaepfer IR. Aberrant lipid metabolism promotes prostate cancer: role in cell survival under hypoxia and extracellular vesicles biogenesis. Int J Mol Sci 2016; 17:E1061. https://doi.org/10.3390/ijms17071061.
doi: 10.3390/ijms17071061
pmid: 27384557
|
| [86] |
Barelli H, Antonny B. Lipid unsaturation and organelle dynamics. Curr Opin Cell Biol 2016; 41:25-32.
|
| [87] |
Fritz V, Benfodda Z, Rodier G, Henriquet C, Iborra F, Avancès C, et al. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol Cancer Ther 2010; 9:1740-54.
|
| [88] |
Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis 2006; 9:230-4.
|
| [89] |
Liu Y, Zuckier L, Ghesani N. Fatty acid rather than glucose metabolism is the dominant bioenergetic pathway in prostate cancer. J Nucl Med 2008; 49(Suppl 1):104P.
|
| [90] |
Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Investig 2010; 120:142-56.
|
| [91] |
Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta 2011; 1807:726-34.
|
| [92] |
Harper ME, Antoniou A, Villalobos-Menuey E, Russo A, Trauger R, Vendemelio M, et al. Characterization of a novel metabolic strategy used by drug-resistant tumor cells. FASEB J 2002; 16:1550-7.
|
| [93] |
Park JH, Vithayathil S, Kumar S, Sung PL, Dobrolecki LE, Putluri V, et al. Fatty acid oxidation-driven Src links mitochondrial energy reprogramming and oncogenic properties in triple-negative breast cancer. Cell Rep 2016; 14:2154-65.
|
| [94] |
Tennakoon JB, Shi Y, Han JJ, Tsouko E, White MA, Burns AR, et al. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1a-mediated metabolic switch. Oncogene 2014; 33:5251-61.
|
| [95] |
Qu Q, Zeng F, Liu X, Wang QJ, Deng F. Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis 2016; 7:e2226. https://doi.org/10.1038/cddis.2016.132.
doi: 10.1038/cddis.2016.132
pmid: 27195673
|
| [96] |
Schlaepfer IR, Rider L, Rodrigues LU, Gijón MA, Pac CT, Romero L, et al. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol Cancer Ther 2014; 13:2361-71.
|
| [97] |
Balaban S, Nassar ZD, Zhang AY, Hosseini-Beheshti E, Centenera MM, Schreuder M, et al. Extracellular fatty acids are the major contributor to lipid synthesis in prostate cancer. Mol Cancer Res 2019; 17:949-62.
|
| [98] |
Watt MJ, Clark AK, Selth LA, Haynes VR, Lister N, Rebello R, et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci Transl Med 2019; 11. https://doi.org/10.1126/scitranslmed.aau5758.
doi: 10.1126/scitranslmed.aav1636
pmid: 31801884
|
| [99] |
Nassar ZD, Aref AT, Miladinovic D, Mah CY, Raj GV, Hoy AJ, et al. Peri-prostatic adipose tissue: the metabolic microenvironment of prostate cancer. BJU Int 2018; 121(Suppl 3):9-21.
|
| [100] |
Tousignant KD, Rockstroh A, Taherian Fard A, Lehman ML, Wang C, McPherson SJ, et al. Lipid uptake is an androgenenhanced lipid supply pathway associated with prostate cancer disease progression and bone metastasis. Mol Cancer Res 2019; 17:1166-79.
|
| [101] |
Laurent V, Guérard A, Mazerolles C, Le Gonidec S, Toulet A, Nieto L, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun 2016; 7:10230. https://doi.org/10.1038/ncomms10230.
pmid: 26756352
|
| [102] |
Pinthus JH, Lu JP, Bidaisee LA, Lin H, Bryskine I, Gupta RS, et al. Androgen-dependent regulation of medium and long chain fatty acids uptake in prostate cancer. Prostate 2007; 67:1330-8.
|
| [103] |
Nath A, Li I, Roberts LR, Chan C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep 2015; 5:14752. https://doi.org/10.1038/srep14752.
pmid: 26424075
|
| [104] |
Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 2017; 541:41-5.
|
| [105] |
Hale JS, Otvos B, Sinyuk M, Alvarado AG, Hitomi M, Stoltz K, et al. Cancer stem cell-specific scavenger receptor CD36 drives glioblastoma progression. Stem Cells 2014; 32:1746-58.
|
| [106] |
Jeon S-M, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012; 485:661-5.
|
| [107] |
Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 2001; 291:2613-6.
|
| [108] |
Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res 2009; 50(Suppl):S138-43. https://doi.org/10.1194/jlr.R800079-JLR200.
|
| [109] |
Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer 2013; 13:227-32.
|
| [110] |
Schlaepfer IR, Nambiar DK, Ramteke A, Kumar R, Dhar D, Agarwal C, et al. Hypoxia induces triglycerides accumulation in prostate cancer cells and extracellular vesicles supporting growth and invasiveness following reoxygenation. Oncotarget 2015; 6:22836-56.
|
| [111] |
Audet-Walsh é, Dufour CR, Yee T, Zouanat FZ, Yan M, Kalloghlian G, et al. Nuclear mTOR acts as a transcriptional integrator of the androgen signaling pathway in prostate cancer. Genes Dev 2017; 31:1228-42.
|
| [112] |
Audet-Walsh é, Vernier M, Yee T, Laflamme C, Li S, Chen Y, et al. SREBF1 activity is regulated by an ar/mtor nuclear axis in prostate cancer. Mol Cancer Res 2018; 16:1396-405.
|
| [113] |
Audet-Walsh é, Yee T, McGuirk S, Vernier M, Ouellet C, St-Pierre J, et al. Androgen-dependent repression of ERRg reprograms metabolism in prostate cancer. Cancer Res 2017; 77:378-89.
|
| [114] |
Heemers HV, Verhoeven G, Swinnen JV. Androgen activation of the sterol regulatory element-binding protein pathway: current insights. Mol Endocrinol 2006; 20:2265-77.
|
| [115] |
Li X, Chen YT, Hu P, Huang WC. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBPregulated metabolic pathways and androgen receptor signaling. Mol Cancer Ther 2014; 13:855-66.
|
| [116] |
Huang WC, Li X, Liu J, Lin J, Chung LWK. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol Cancer Res 2012; 10:133-42.
|
| [117] |
Huang WC, Zhau HE, Chung LWK. Androgen receptor survival signaling is blocked by anti-beta2-microglobulin monoclonal antibody via a MAPK/lipogenic pathway in human prostate cancer cells. J Biol Chem 2010; 285:7947-56.
|
| [118] |
Ettinger SL, Sobel R, Whitmore TG, Akbari M, Bradley DR, Gleave ME, et al. Dysregulation of sterol response elementbinding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res 2004; 64:2212-21.
|
| [119] |
Sharma NL, Massie CE, Ramos-Montoya A, Zecchini V, Scott HE, Lamb AD, et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell 2013; 23:35-47.
|
| [120] |
Pullar B, Shah N. Prostate cancer. Surgery (Oxford) 2016; 34:505-11.
|
| [121] |
Qi J, Tripathi M, Mishra R, Sahgal N, Fazli L, Ettinger S, et al. The E3 ubiquitin ligase Siah2 contributes to castrationresistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer Cell 2013; 23:332-46.
|
| [122] |
Alsinnawi M, Zhang A, Bianchi-Frias D, Burns J, Cho E, Zhang X, et al. Association of prostate cancer SLCO gene expression with Gleason grade and alterations following androgen deprivation therapy. Prostate Cancer Prostatic Dis 2019; 22:560-8.
|
| [123] |
Shafi AA, Putluri V, Arnold JM, Tsouko E, Maity S, Roberts JM, et al. Differential regulation of metabolic pathways by androgen receptor (AR) and its constitutively active splice variant, AR-V7, in prostate cancer cells. Oncotarget 2015; 6. 31997-2012.
|
| [124] |
Han W, Gao S, Barrett D, Ahmed M, Han D, Macoska JA, et al. Reactivation of androgen receptor-regulated lipid biosynthesis drives the progression of castration-resistant prostate cancer. Oncogene 2018; 37:710-21.
|
| [125] |
Kong Y, Cheng L, Mao F, Zhang Z, Zhang Y, Farah E, et al. Inhibition of cholesterol biosynthesis overcomes enzalutamide resistance in castration-resistant prostate cancer (CRPC). J Biol Chem 2018; 293:14328-41.
|
| [126] |
Flaig TW, Salzmann-Sullivan M, Su LJ, Zhang Z, Joshi M, Gijón MA, et al. Lipid catabolism inhibition sensitizes prostate cancer cells to antiandrogen blockade. Oncotarget 2017; 8:56051-65.
|
| [127] |
Zadra G, Ribeiro CF, Chetta P, Ho Y, Cacciatore S, Gao X, et al. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc Natl Acad Sci USA 2019; 116:631-40.
|
| [128] |
Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014; 371:1028-38.
|
| [129] |
Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013; 155:1309-22.
|
| [130] |
Sahu B, Laakso M, Pihlajamaa P, Ovaska K, Sinielnikov I, Hautaniemi S, et al. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res 2013; 73:1570-80.
|
| [131] |
Li J, Alyamani M, Zhang A, Chang KH, Berk M, Li Z, et al. Aberrant corticosteroid metabolism in tumor cells enables GR takeover in enzalutamide resistant prostate cancer. Elife 2017; 6:e20183. https://doi.org/10.7554/eLife.20183.
pmid: 28191869
|
| [132] |
Wang JC, Gray NE, Kuo T, Harris CA. Regulation of triglyceride metabolism by glucocorticoid receptor. Cell Biosci 2012; 2:19. https://doi.org/10.1186/2045-3701-2-19.
pmid: 22640645
|
| [133] |
Zhang Z, Hou X, Shao C, Li J, Cheng JX, Kuang S, et al. Plk1 inhibition enhances the efficacy of androgen signaling blockade in castration-resistant prostate cancer. Cancer Res 2014; 74:6635-47.
|
| [134] |
Yuan Y. Spatial heterogeneity in the tumor microenvironment. Cold Spring Harb Perspect Med 2016; 6:a026583. https://doi.org/10.1101/cshperspect.a026583.
pmid: 27481837
|
| [135] |
Berglund E, Maaskola J, Schultz N, Friedrich S, Marklund M, Bergenstr?hle J, et al. Spatial maps of prostate cancer transcriptomes reveal an unexplored landscape of heterogeneity. Nat Commun 2018; 9:2419. https://doi.org/10.1038/s41467-018-04724-5.
pmid: 29925878
|
| [136] |
Curtius K, Wright NA, Graham TA. An evolutionary perspective on field cancerization. Nat Rev Cancer 2018; 18:19-32.
|
| [137] |
De Vivar AD, Sayeeduddin M, Rowley D, Cubilla A, Miles B, Kadmon D, et al. Histologic features of stromogenic carcinoma of the prostate (carcinomas with reactive stroma grade 3). Hum Pathol 2017; 63:202-11.
|
| [138] |
Randall EC, Zadra G, Chetta P, Lopez BGC, Syamala S, Basu SS, et al. Molecular characterization of prostate cancer with associated Gleason Score using mass spectrometry imaging. Mol Cancer Res 2019; 17:1155-65.
|
| [139] |
Morse N, Jamaspishvili T, Simon D, Patel PG, Ren KYM, Wang J, et al. Reliable identification of prostate cancer using mass spectrometry metabolomic imaging in needle core biopsies. Lab Investig 2019; 99:1561-71.
|
| [1] |
Renee E. Vickman,Omar E. Franco,Daniel C. Moline,Donald J. Vander Griend,Praveen Thumbikat,Simon W. Hayward. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A review[J]. Asian Journal of Urology, 2020, 7(3): 191-202. |
| [2] |
Simeng Wen,Yuanjie Niu,Haojie Huang. Posttranslational regulation of androgen dependent and independent androgen receptor activities in prostate cancer[J]. Asian Journal of Urology, 2020, 7(3): 203-218. |
| [3] |
Ieva Eringyte,Joanna N. Zamarbide Losada,Sue M. Powell,Charlotte L. Bevan,Claire E. Fletcher. Coordinated AR and microRNA regulation in prostate cancer[J]. Asian Journal of Urology, 2020, 7(3): 233-250. |
| [4] |
Yezi Zhu,Jun Luo. Regulation of androgen receptor variants in prostate cancer[J]. Asian Journal of Urology, 2020, 7(3): 251-257. |
| [5] |
Ramesh Narayanan. Therapeutic targeting of the androgen receptor (AR) and AR variants in prostate cancer[J]. Asian Journal of Urology, 2020, 7(3): 271-283. |
| [6] |
Abhishek Tripathi,Shilpa Gupta. Androgen receptor in bladder cancer: A promising therapeutic target[J]. Asian Journal of Urology, 2020, 7(3): 284-290. |
| [7] |
Pei Zhao,Yezi Zhu,Liang Cheng,Jun Luo. Detection of androgen receptor (AR) and AR-V7 in small cell prostate carcinoma: Diagnostic and therapeutic implications[J]. Asian Journal of Urology, 2019, 6(1): 109-113. |
| [8] |
Cameron M. Armstrong,Allen C. Gao. Current strategies for targeting the activity of androgen receptor variants[J]. Asian Journal of Urology, 2019, 6(1): 42-49. |
| [9] |
Lingfan Xu,Junyi Chen,Weipeng Liu,Chaozhao Liang,Hailiang Hu,Jiaoti Huang. Targeting androgen receptor-independent pathways in therapy-resistant prostate cancer[J]. Asian Journal of Urology, 2019, 6(1): 91-98. |
| [10] |
Jun Luo. Non-invasive actionable biomarkers for metastatic prostate cancer[J]. Asian Journal of Urology, 2016, 3(4): 170-176. |
| [11] |
Jin Xu, Yun Qiu. Role of androgen receptor splice variants in prostate cancer metastasis[J]. Asian Journal of Urology, 2016, 3(4): 177-184. |
| [12] |
Zheng Cao, Natasha Kyprianou. Mechanisms navigating the TGF-β pathway in prostate cancer[J]. Asian Journal of Urology, 2015, 2(1): 11-18. |
| [13] |
Styliani Karanika, Theodoros Karantanos, Jianhua Yin, Likun Li, Timothy C. Thompson. Novel anti-androgen receptor signaling agents: understanding the mechanisms of resistance[J]. Asian Journal of Urology, 2014, 1(1): 28-37. |
|
|
|
|




