|
|
|
| Comparative assessment of efficacy and safety of different treatment for de novo overactive bladder children: A systematic review and network meta-analysis |
Shi Qiu1,Siwei Bi1,Tianhai Lin1,Zhuheng Wu,Qi’an Jiang,Jiwen Geng,Liangren Liu,Yige Bao,Xiang Tu,Mingjing He,Lu Yang( ),Qiang Wei( ),Qiang Wei( ) )
|
| Department of Urology, Institute of Urology, West China Hospital of Sichuan University, Chengdu, China |
|
|
|
|
Abstract Objective: To compare these managements focusing on the efficacy and safety to treat overactive bladder (OAB) in children through network meta-analysis (NMA). Methods: We searched PubMed, Embase, the Cochrane Library Central Register of Controlled Trials (CENTRAL) and the reference lists up to May 1st, 2017. Data from eligible randomized controlled trails (RCT) studies including three different treatment options were extracted. The primary outcome was maximal voiding volume (MVV). We performed pairwise meta-analyses by random effects model and NMA by Bayesian model. We used the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework to assess the quality of evidence contributing to each network estimate. Results: Six RCTs (462 patients) comparing three different interventions fulfilled the inclusion criteria. A low risk of bias was shown for the majority of the study items. The results of NMA showed that compared with antimuscarinic drugs, Parasacral transcutaneous electrical nerve stimulation was associated with significant improvement in the MVV (mean difference [MD] = 58.50, 95% confidential interval [CI]: 45.95-69.52), followed by urotherapy group (MD = 21.03, 95% CI: 11.85-29.97). When it comes to the constipation, antimuscarinic drugs exerted significant benefit than PTENS (odds ratio [OR]: 0.22, 95% CI: 0.01-0.46). No significant difference was found between other treatments. Conclusion: Compared with antimuscarinic drugs, PTENS was associated with significant better efficacy considering MVV, but more constipation events in de novo OAB children. Antimuscarinic drugs showed remarkably better efficacy considering MVV and comparable safety profile compared with urotherapy. Clinicians should take all known safety and compliance of patients into account when choosing an optimal strategy.
|
|
Received: 15 August 2017
Available online: 13 April 2019
|
|
Corresponding Authors:
Lu Yang,Qiang Wei
E-mail: wycleflue@163.com;weiqiang163163@163.com
|
|
|
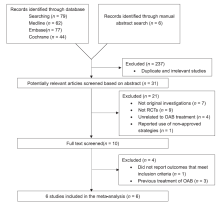
|
|
Flow chart of study identification and selection procedure. OAB, overactive bladder; RCTs, randomized controlled trails.
|

| Author | Publish year | Country | Intervention group 1 (sample size) | Intervention group 2 (sample size) | stimulation frequency | Stimulation duration (min/time) | Treatment duration (week) | Treatment frequency | | de Paula et al. [17] | 2017 | Brazil | PTENS (8) | Sham stimulation (8) | 10 Hz | 20 | 20 | Once a week | | Lordêlo et al. [18] | 2010 | Brazil | PTENS (21) | Scapular stimulation (16) | 10 Hz | 20 | 6 | Three times a week | | Sillén et al. [19] | 2014 | Sweden | PTENS and urotherapy (30) | Urotherapy (32) | 10 Hz | 20 | 12 | Twice daily | | Quintiliano et al. [20] | 2015 | Brazil | PTENS and placebo (13) | Antimuscainic (oxybutynin) and sham electric stimulation (15) | 10 Hz | 20 | 6 | Three times a week | | Marschall-Kehrel et al. [21] | 2009 | Germany | Antimuscarinic (propiverine) (87) | Placebo (84) | NA | NA | 8 | Twice daily | | Newgreen et al. [22] | 2016 | Netherlands | Antimuscarinic (Solifenacin) (73) | Placebo (73) | NA | NA | 12 | Once daily |
|
|
The main characteristics of the included RCTs.
|
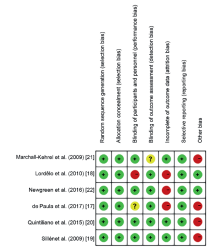
|
|
Risk of bias assessments within studies.
|
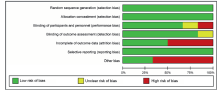
|
|
Risk of bias assessments for each study.
|
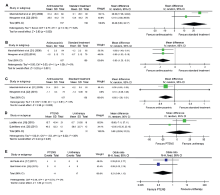
|
|
Forest plot for pairwise meta-analysis. (A) Change in maximal voiding volume; (B) Voiding frequency; (C) Incontinence episodes; (D) Change in average voiding volume change; (E) Constipation. The size of the boxes corresponds to each study's weight. CI, confidential interval; PTENS, parasacral transcutaneous electrical nerve stimulation. IV, Inverse variance; M-H, Mantel-Haenszel.
|

| Comparison | No. of participants | No. of trials | Pairwise meta-analysis mean difference/odd ratios (95% CI) | p-Value | Heterogeneity, I2 | Network meta-analysis, mean difference/odds ratios (95% CrI) | Quality of evidence | Downgraded reason | | Maximal voiding volume | | PTENS vs. urotherapy | 196 | 2 | NA | NA | NA | 37.46 (28.27, 45.24) | ⊕⊕○○ low | Inconsistency and imprecision | | Antimuscarinic vs. urotherapy | 307 | 2 | 20.49 (6.80, 34.17) | 0.1 | 64% | 21.03 (11.85, 29.97) | ⊕⊕⊕○ moderate | heterogeneity | | PTENS vs. antimuscarinic | 239 | 0 | NA | NA | NA | 58.50 (45.95, 69.52) | ⊕⊕○○ low | Heterogeneity and imprecision | | Voiding frequency | | PTENS vs. urotherapy | 196 | 2 | NA | NA | NA | 0.425 (-2.21, 2.76) | ⊕⊕○○ low | Inconsistency and imprecision | | Antimuscarinic vs. urotherapy | 307 | 2 | -0.80 (-1.29, -0.31) | 1 | 0 | 1.09 (-1.18, 3.40) | ⊕⊕○○ low | Inconsistency and imprecision | | PTENS vs. antimuscarinic | 239 | 0 | NA | NA | NA | 0.67 (-2.213, 3.71) | ⊕⊕○○ low | Heterogeneity and imprecision | | Incontinence episodes | | PTENS vs.urotherapy | 219 | 1 | NA | NA | NA | 0.13 (-4.88, 5.44) | ⊕⊕○○ low | Inconsistency and imprecision | | Antimuscarinic vs. urotherapy | 349 | 2 | -0.30 (-0.54, -0.05) | 0.81 | 0 | 0.23 (-3.41, 3.74) | ⊕⊕○○ low | Inconsistency and imprecision | | PTENS vs. antimuscarinic | 190 | 0 | NA | NA | NA | 0.09 (-6.79, 6.59) | ⊕⊕○○ low | Heterogeneity and imprecision | | Constipation | | PTENS vs. urotherapy | 29 | 1 | 0.21 (0.04, 1.12) | 0.51 | 0 | 0.38 (0.01, 6.85) | ⊕⊕⊕○ moderate | Heterogeneity | | Antimuscarinic vs. urotherapy | 23 | 0 | NA | NA | NA | 0.15 (0.25, 3.82) | ⊕⊕○○ low | Heterogeneity and imprecision | | PTENS vs. antimuscarinic | 36 | 1 | NA | NA | NA | 0.22 (0.01, 0.46) | ⊕⊕○○ low | Inconsistency and imprecision |
|
|
Summary effect size of pairwise and network meta-analysis.
|
| [1] |
Austin PF, Bauer SB, Bower W, Chase J, Franco I, Al PH , et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the Standardization Committee of the International Child. Neurourol Urodyn 2016; 35:471-81.
|
| [2] |
Franco I . Overactive bladder in children. Nat Rev Urol 2016; 13:520-32.
|
| [3] |
Hellstrom A, Hanson E, Hansson S, Hjalmas K, Jodal U . Micturition habits and incontinence at age 17dreinvestigation of a cohort studied at age 7. Br J Urol 1995; 76:231-4.
|
| [4] |
Swithinbank LV, Brookes ST, Shepherd AM, Abrams P . The natural history of urinary symptoms during adolescence. Br J Urol 1998; 81(suppl 3):90-3.
|
| [5] |
Bartoli S, Aguzzi G, Tarricone R . Impact on quality of life of urinary incontinence and overactive bladder: a systematic literature review. Urology 2010; 75:491-500.
|
| [6] |
Landgraf JM, Abidari J, Cilento BGJ, Cooper CS, Schulman SL, Ortenberg J . Coping, commitment, and attitude: quantifying the everyday burden of enuresis on children and their families. Pediatrics 2004; 113:334-44.
|
| [7] |
Joinson C, Heron J, von Gontard A . Psychological problems in children with daytime wetting. Pediatrics 2006; 118:1985e93.
|
| [8] |
Ramsay S, Bolduc S . Overactive bladder in children. Can Urol Assoc J. 2017; 11:S74-9.
|
| [9] |
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C , et al. The PRISMA extension statement for reporting of systematic reviews incorporating network metaanalyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162:777-84.
|
| [10] |
Higgins J, Green S . Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Cochrane Collab 2011.
|
| [11] |
DerSimonian R, Laird N . Meta-analysis in clinical trials. Contr Clin Trials 1986; 7:177-88.
|
| [12] |
Salanti G, Higgins JP, Ades A, Ioannidis JP . Evaluation of networks of randomized trials. Stat Methods Med Res 2007; 17:279-301.
|
| [13] |
Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR . Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012; 3:98-110.
|
| [14] |
Higgins JPT, Thompson SG, Deeks JJ, Altman DG . Measuring inconsistency in meta-analyses. BMJ Br Med J 2003; 327:557-60.
|
| [15] |
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso- Coello P , et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924-6.
|
| [16] |
Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JPT . Evaluating the quality of evidence from a network meta-analysis. PLoS One 2014;9:e99682.
|
| [17] |
de Paula LIDS, de Oliveira LF, Cruz BP, de Oliveira DM, Miranda LM, de Moraes Ribeiro M , et al. Parasacral transcutaneous electrical neural stimulation (PTENS) once a week for the treatment of overactive bladder in children: a randomized controlled trial. J Pediatr Urol 2017; 13:263. e1-6.
|
| [18] |
Lorde?lo P, Teles A, Veiga ML, Correia LC, Barroso U . Transcutaneous electrical nerve stimulation in children with overactive bladder: a randomized clinical trial. J Urol 2010; 184:683-9.
|
| [19] |
Sillén U, Arwidsson C, Doroszkiewicz M, Antonsson H, Jansson I, St?lklint M , et al. Effects of transcutaneous neuromodulation (TENS) on overactive bladder symptoms in children: a randomized controlled trial. J Pediatr Urol 2014; 10:1100-5.
|
| [20] |
Quintiliano F, Veiga ML, Moraes M, Cunha C, De Oliveira LF, Lordelo P , et al. Transcutaneous parasacral electrical stimulation vs oxybutynin for the treatment of overactive bladder in children: a randomized clinical trial. J Urol 2015; 193:1749-53.
|
| [21] |
Marschall-Kehrel D, Feustel C, Persson de Geeter C, Stehr M, Radmayr C, Sillén U , et al. Treatment with propiverine in children suffering from nonneurogenic overactive bladder and urinary incontinence: results of a randomized placebocontrolled phase 3 clinical trial. Eur Urol 2009; 55:729-38.
|
| [22] |
Newgreen D, Bosman B, Hollestein-Havelaar A, Dahler E, Besuyen R, Sawyer W , et al. Solifenacin in children and adolescents with overactive bladder: results of a phase 3 randomised clinical trial. Eur Urol 2017; 71:483-90.
|
| [23] |
Walsh IK, Johnston RS, Keane PF . Transcutaneous sacral neurostimulation for irritative voiding dysfunction. Eur Urol 1999; 35:192-6.
|
| [24] |
Hoebeke P, Renson C, Petillon L, Vande Walle J, De Paepe H . Percutaneous electrical nerve stimulation in children with therapy resistant nonneuropathic bladder sphincter dysfunction: a pilot study. J Urol 2002; 168:2605-8.
|
| [25] |
Bower WF, Moore KH, Adams RD . A pilot study of the home application of transcutaneous neuromodulation in children with urgency or urge incontinence. J Urol 2001; 166:2420-2.
|
| [26] |
Yik YI, Clarke MCC, Catto-Smith AG, Robertson VJ, Sutcliffe JR, Chase JW , et al. Slow-transit constipation with concurrent upper gastrointestinal dysmotility and its response to transcutaneous electrical stimulation. Pediatr Surg Int 2011; 27:705-11.
|
| [27] |
Ismail KA, Chase J, Gibb S, Clarke M, Catto-Smith AG, Robertson VJ , et al. Daily transabdominal electrical stimulation at home increased defecation in children with slowtransit constipation: a pilot study. J Pediatr Surg 2009; 44:2388-92.
|
| [1] |
Kenji Omae,Noriaki Kurita,Sei Takahashi,Shingo Fukuma,Yosuke Yamamoto,Shunichi Fukuhara,The Sukagawa Study Group. Association of advanced glycation end-product accumulation with overactive bladder in community-dwelling elderly: A cross-sectional Sukagawa study[J]. Asian Journal of Urology, 2021, 8(2): 189-196. |
| [2] |
Alberto Abrate,Andrea Gregori,Alchiede Simonato. Lingual mucosal graft urethroplasty 12 years later: Systematic review and meta-analysis[J]. Asian Journal of Urology, 2019, 6(3): 230-241. |
| [3] |
Martin Slovak, Christopher R. Chapple, Anthony T. Barker. Non-invasive transcutaneous electrical stimulation in the treatment of overactive bladder[J]. Asian Journal of Urology, 2015, 2(2): 92-101. |
| [4] |
Bilal Chughtai, Aizaz Ali, Claire Dunphy, Steven A. Kaplan. Effect of phosphodiesterase inhibitors in the bladder[J]. Asian Journal of Urology, 2015, 2(1): 33-37. |
|
|
|
|




